Quantum Mechanics
Electron-quark interactionsFrom a conventional physics perspective the internal makeup of protons and
neutrons is considered unimportant because electrons only ‘see’ the net charge
contained within a nucleus. But the ideas discussed in earlier chapters point to a
different approach. Namely that we shouldn’t only consider net charge but also
individual charge components because their movement affects the magnitude and direction of
forces applied.
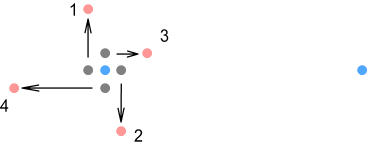
Here the up-quarks are shown orbiting in the same plane as the electron. Due to the nature of VDCL and sideways forces, as the quarks move through their orbit they exert varying degrees of force in different directions. We’ll consider four points in the orbit; located at the left, right, top and bottom positions and numbered 1,2,3,4 respectively.
First the left and right positions (numbered 1 and 2). As the position 1
quark moves up it will exert a sideways force on the electron in the downward direction.
Conversely, as the position 2 quark moves down it will exert that same force but in the
upward direction. As a result the two sideways forces cancel and can be ignored. Now for
positions 3 and 4 (top and bottom). As position 4 moves toward the electron its force will
decrease; and as position 3 moves away its force will increase. If we were to examine the
net contribution from all up-quark positions (going around the circle), we would find an
overall increase in the proton’s attraction on the electron.
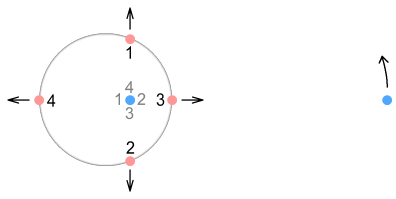
Virtual-current quarksIn the second chapter on sideways forces (part 2), we looked at something called a virtual-current particle. This is the hypothetical position of a source particle that is seen by a target, and the position we base force calculations on. For situations where the source particle is non-accelerating, i.e. moving in a straight line at constant speed, the virtual-current position can be ignored because it is the same as the actual position. In fact the virtual current position can be ignored in almost every low-velocity situation as it corresponds closely to the actual position. The proton however is neither a low-velocity nor a zero-acceleration situation. With its quarks cornering at high speed around tight circles, the proton is a situation in which virtual-current particles become important. The below diagram explains this.
Here the previous situation is repeated but this time showing both phantom and virtual-current particles. The phantom particles, shown in grey, correspond to the ones shown earlier (although closer to the nucleus here). It takes some time for the signal from an up-quark (the source) to reach the electron (the target) and in that time the quark, if it had been moving in a straight line, would have moved well outside the boundaries of the proton. For example the quarks moving up and down (positions 1 and 2) have virtual current particles far above and below, and the left and right (positions 3 and 4) have virtual particles well in front and behind the proton radius. The left-moving quark is very far out because its signal toward the electron moves less than light speed and takes longer to get there. Let’s repeat this diagram but this time using a full circle:
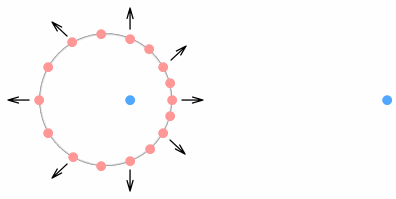
Here we see the tiny circle of the proton orbit has expanded into a large
virtual-current circle (the phantoms are not shown). This is somewhat surprising because
it shows that what the electron sees is not a tiny ball of closely-packed charges but a
‘cloud’ of positive charges orbiting at a large distance from a central negative
charge. Although not orbiting in a normal sense because their velocity is always pointing
outward. More specifically, what the electron sees is a ring of positive charges expanding
away from the negative quark. Except that the ring never gets larger. To make an analogy,
this ring is like a motion-blurred photograph of a speeding car – it appears to be
moving forward but its position never changes.
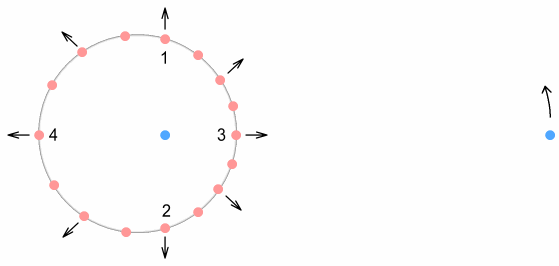
This time the electron is shown in motion around the proton. It orbits at
a fixed distance, and is moving upward at this moment. Positions 3&4, corresponding to
the up-quarks at the top and bottom of the down-quark, have their virtual-current
positions to the left and right. Because of the direction of motion, they will cause a net
increase on the force of the electron. As pointed out earlier however, this will not do
anything to the electron orbit other than require it to move faster.
What will happen is that the positive ring will become larger as the
electron moves further away. This is because the field takes longer to reach its target,
thus moving the virtual-current charges further from their source. Of course the ring does
not move at all; that’s just how the electron sees it. If there were several
electrons surrounding the proton, each would see a different virtual ring of differing
size and position.
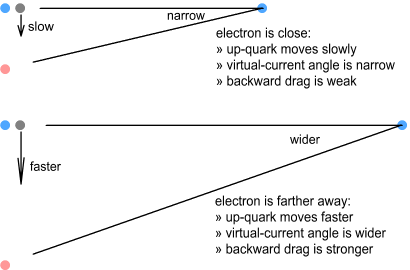
Orbital speed of quarksAs we’ve seen, the size of the virtual ring depends on the distance
to the electron. It also depends on the speed the up-quarks orbit the central down-quark
– the faster they go, the larger the ring. As described in the chapter on nucleon structure, the speed these quarks move is
determined by the size, mass and charge distribution of the quarks (both types). But could
their speed also be influenced by the electron nearby? One would expect so. As the
orbiting quarks moved back and forth in the electron’s field they should experience a
net drag. The result of this would be to make the quarks orbit at a lower frequency when
the electron is nearby and at a higher frequency when the electron is further away.
What we will have then is monatomic hydrogen – a stand-alone hydrogen atom. The electron is now orbiting at a fixed distance and is doing so at a set frequency. When viewed from the side, the electron appears as a sinusoidal oscillator. Thus it radiates this frequency into the surrounding environment and will be seen as a pure (fixed) frequency emission line on a spectrometer.
Hydrogen at different frequenciesThe hydrogen atom described above emitted radiation at a fixed frequency.
In reality however what we observe are a number of discrete frequencies radiating from
excited hydrogen. Could the above model also account for that? In order for the electron
to orbit at a number of stable frequencies we need to show that there are multiple
altitudes at which both the net radial (outward) force and the net sideways force on the
electron are zero.
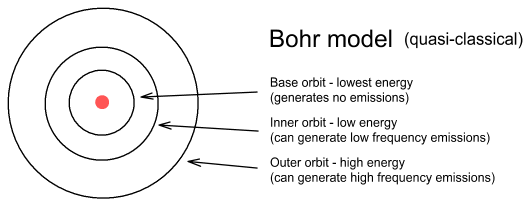
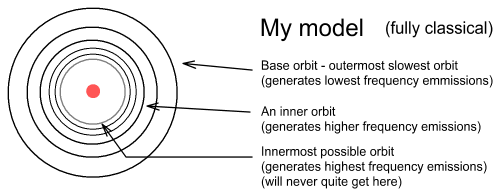
In the Bohr model there is no upper limit to the altitude electrons can orbit. In my model there is both an upper and lower limit and the distance between successive higher frequency orbits become ever smaller.
Monatomic Hydrogen StabilityThe configuration described above suggests monatomic hydrogen is stable and its electron could continue orbiting like this forever. In reality however the constant radiation such an atom emits would disturb nearby atoms, causing them to emit radiation that disturbs the original atom in turn. As a result the electron orbit would eventually break down, causing its emission to cease. This is consistent with observation which tells us that emissions from radiating atoms are brief.
The Helium AtomIn the earlier atomic orbits
chapter one model considered for helium was to have two electrons situated on opposite
sides of the nucleus. In this way the sinusoidal radiation emitted from one orbiting
electron would be 180 degrees out of phase with the other and would cancel. This model
however was rejected on the grounds that (a) the net sideways force on each electron was
promotional, which would cause it to spiral outward and (b) an electron might drop to a
slightly lower orbit altitude, get underneath the other and eject it.
As described in the chapter on atomic nuclei, the helium nucleus consists of two protons and (usually) two neutrons arranged end-to-end and orbiting in the same direction. So it presents the same basic charge profile as a hydrogen nucleus. Like the hydrogen nucleus, the helium quarks will orbit in the same plane as the electrons. What helium electrons will see is a set of up-quarks orbiting negative charges, and these will determine the possible electron orbit altitudes.
Hydrogen MoleculesHydrogen’s most stable and common formation is as a molecule
consisting of two hydrogen atoms bound in some manner. In the atomic orbits chapter I suggested the two
atoms sit side-by-side, their electrons orbiting in the same plane and in opposite
directions. In this way the electrons would cancel each other’s signal and also
create a bond between the two atoms. As mentioned above however, this model failed a
computer simulation test, which showed that electrons ‘despise’ running in
opposite directions because the VDCL force between them becomes too great. Using the new
knowledge about the rotating quarks then, can we create a stable model for hydrogen?
Here the protons are side-by-side and the electrons orbit between them. This is like the neutron model which had the up-quarks orbiting between the negative charges. The electrons attract the protons and draw them together and the protons repel each other. This creates a stable formation.
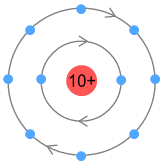
Higher order elementsSchoolbook chemistry tells us that atomic electrons are arranged in multiple shells, with each shell representing an orbit holding one or more electrons. The first of these shells holds either one or two electrons and caters for hydrogen and helium. The next shell holds up to eight and covers the second row of the period table: lithium to neon. We could draw the neon atom looking like it does in the classical sense:
Here both shells are circular paths sitting a fixed distance from the
nucleus. Inside a shell the electrons are evenly spaced. To prevent VDCL repulsion both
shells need to orbit in the same direction, although the inner shell electrons will move
faster. The fixed orbit of the inner shell electrons will influence the electrons in the
outer shell and likewise ‘quantize’ them to orbit at a fixed altitude(s). The
number of electrons in a shell would need to be multiple of 2, such that a pair orbiting
opposite each other would cancel each other’s signal and prevent. But why does the
second shell hold 8 and not 2, 4, 6, 10 etc.? That’s difficult to answer. Perhaps
there is sufficient space to hold 8 electrons and no more. |