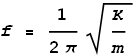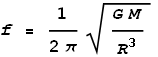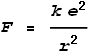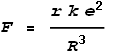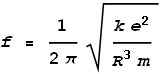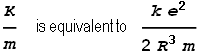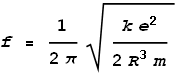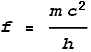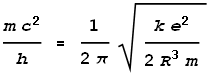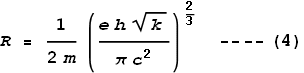Determining the Electron Structure
The classical electron radiusBefore going further some points need to be made about something called
the ‘classical electron radius’. This is a calculated radius based on an
assumption that the mass-energy potential of an electron is fully contained within a
certain radius [2]. It has a value of 2.82x10-15 m.
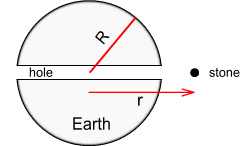
Falling through the EarthImagine you bored a hole through the Earth – straight down through its centre and out the other side. Next you dropped a stone at the entrance. The stone would fall rapidly at first, gaining in speed until it reached terminal velocity. It would then continue toward the Earth’s centre and past it. Once beyond the mid-point it would decelerate as it neared the other side. The stone would then oscillate several times until it came to rest at the Earth’s centre.
Let’s assume instead that there was no air and the stone was able to
move all the way from one side of the Earth to the other and back again, much like a
pendulum. What would be its precise motion of oscillation, and how long would it take for
a full ‘swing’?
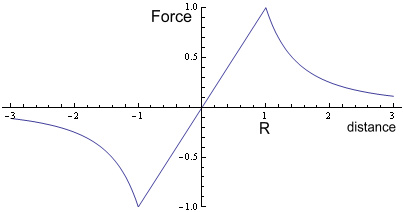
Where M is mass of Earth, m is mass of an object (the stone in this case) and r is distance from the centre. Below the surface the force follows a linear function:
Where R is the radius of the Earth. If we were to plot this it would show (setting, G=M=m=R = 1, and including force direction):
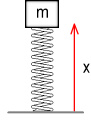
A simple spring oscillatorTo understand what this means we’ll look at a simple spring oscillator.
Above we see an object with mass m attached to a spring.
The spring has a stiffness of K meaning that, e.g. if K
was 5 N/m then every 1 metre of stretch would require 5 Newtons of force. Assuming we are
in a zero-gravity environment and the mass could move below the baseline, what function
would describe the position of the object with respect to time?
Where x(t) is the height of the object at any time t
and x’’(t) is its double derivative – i.e. its
acceleration.
Where A is the initial height and we assume the object to be initially motionless. In other words, the object oscillates as a pure sine wave. The frequency of oscillation will be:
And the period of oscillation will be the reciprocal: T=1/f.
Inserting values for G, M and R, and taking the reciprocal we get a period of 5060 seconds, or 1 hour 24 minutes. Thus it will take just under one and a half hours for the stone to return to its starting point.
Falling through an electronWhat does any of this have to do with electron-positron interactions?
Well, gravity is a good analogy to the electric force because it varies in inverse
proportion to distance. If we think of an electron as spherical and assume that its charge
is uniformly distributed then its force function (exerted on a positron) would be very
similar to Earth’s gravitational field, namely:
Where k is Coulomb’s constant, e is an electron’s charge and r is distance from centre. Inside the electron the force would follow a linear function:
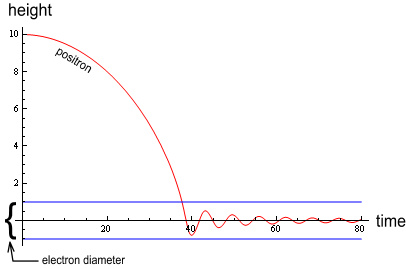
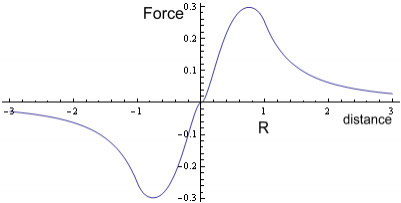
Where R is the electron’s radius. If the positron were to oscillate only within the electron this would produce a sine-wave motion. Does it oscillate within the electron? The below chart shows a simulation of the event:
In this diagram a positron (red line) falls from a height equal to 10
electron radii. As can be seen the VDCL
(Velocity Dependent Coulombs Law) causes much
dampening during the initial fall, causing the oscillations to lie fully within the
electron (blue lines). Thus we can calculate the motion of oscillation based on the forces
within the electron and ignore the force function outside it.
Meaning that frequency of oscillation will be:
Solving the two body problemAt this point it would be tempting to plug values in and calculate a value of R that corresponded to the observed frequency. However there’s something else that needs to be taken into account; namely that is we are not dealing with a stone falling through the Earth. Rather, we are dealing with two earth’s falling through each other, or in this case, an electron and positron oscillating through each other.
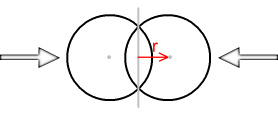
Determining the force function for two spheres is going to be more complicated. As it happens, the force function will be same as the normal Coulomb force when the spheres are outside each other, i.e. not overlapping. When they are overlapping the function becomes very complex but here’s what it looks like:
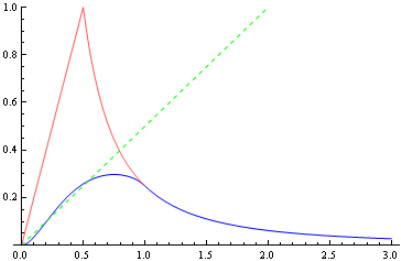
The x-axis represents the distance from the mid-point of the two spheres because this is the point that the spheres will be mirrored on. As can be seen the force function is ‘rounder’ than the single sphere situation and is not a straight line. Here is a comparison between the two functions:
The blue line is the two-sphere force function, red is the single sphere situation (shown for comparison), and the green dotted line represents half the gradient of the red line. As can be seen the force function for when the sphere’s overlap closely approximates half the strength of the single sphere situation. Thus we can relate this back to our simple spring oscillator and find that:
Meaning that frequency of oscillation will be:
We know that this frequency must correspond to the one calculated from quantum mechanical and relativistic energy equations, namely:
Meaning that:
Where h is Planck’s constant. Next we match these two frequencies together:
Isolating the radius term we get:
Substituting the standard values for e, h, k and c, we find that radius of an electron R comes to: 5.94x10-14 m.
DiscussionThis is disappointing. We wanted a radius at least a hundred or so times
smaller than a proton. Instead we calculate one fifty times larger. It’s hard to
believe that this number reflects the true electron radius. Is the frequency correct?The first possibility is a question of frequency. The frequency of gamma
radiation arising from electron-positron annihilation is quoted as 1.24x1020
Hz. But this is not a measurement, only a calculation based on the physical constants;
namely frequency = mc2/h. It is not possible to measure
frequencies this high, e.g. by electronic counting, because we have no instrument that can
keep up with the oscillation rate. A radio antenna comparisonSome interesting analogies can be drawn between the high frequencies of
gamma rays and the low frequencies of radio transmission. We know that a radio
receiver’s antenna is most effective when its length matches half the wavelength of
the transmission frequency. For example a Wi-Fi wireless computer network operating on 2.4
GHz uses antennas around 6 cm in length because the wavelength of that frequency is 12.5
cm. Energy/frequency conversion formulaWhen determining the gamma ray frequency, we did so from its energy using
the formula E=hf; where E is energy, f
is frequency and h is Planck’s constant. This formula is used to
calculate the energy of a photon based on frequency, especially photons created when
electrons transit between atomic shells.
So what is the answer?Unfortunately this lack of information regarding frequency has left us unable to determine the size of an electron. However jumping ahead to the information given in chapters on particle physics and nucleon structure it should be possible to estimate an electron’s diameter based on a proton’s. Without giving away the story in advance, based on this I estimate the radius of an electron to be approximately 10-16 metres.
ConclusionsThe fixed-frequency radiation emitted during electron-positron
annihilation indicates that an electron has a spherical structure and its charge is
uniformly distributed within. |